Medical Marijuana and Multiple Sclerosis

We have all been made aware of the ongoing, red tape-laden political drama that has been taking place over the issue of medical marijuana in the United States. We sit in a strange, temporary position at this point as a nation, with almost half of the 50 states legalizing the use of medical marijuana, several legalizing recreational use, and most still deeming it utterly illegal.
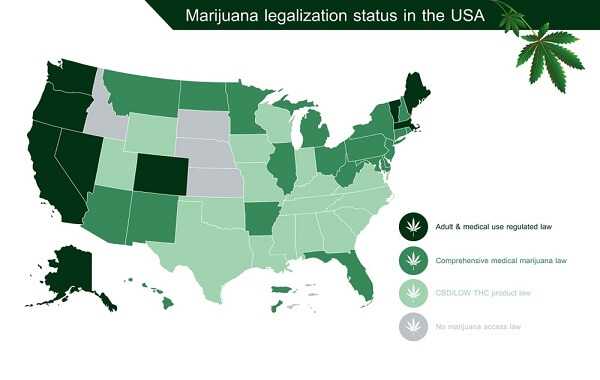
From a federal standpoint, marijuana of any kind for any purpose is considered illegal. However, the states have been granted some leeway, allowing them to approve use depending on the decision of voters. In 1996, California became the first state to approve the use of medical cannabis. There are currently 23 states, plus Washington, DC and Guam, allowing the use of medical cannabis to some extent. The laws are incredibly complex and difficult to understand, with some states allowing only limited use of products containing CBD (the anti-inflammatory component of cannabis), without the mind-altering component, THC. Both Colorado and Washington State have legalized all use of cannabis, including recreational use.
All drugs are listed on a “schedule” system by the DEA (drug enforcement administration.) These schedules range from 1 to 5, and classify drugs considered to have the potential for addiction or dependence. Drugs included on this list include opiate pain medications, anti-anxiety medications such as Ativan and Valium, and ADHD medications (stimulants) such as Adderall. Here is the DEA’s description of these schedule categories:
Schedule I Controlled Substances
Substances in this schedule have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse.
Some examples of substances listed in Schedule I are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), peyote, methaqualone, and 3,4-methylenedioxymethamphetamine (“Ecstasy”).

Schedule II/IIN Controlled Substances (2/2N)
Substances in this schedule have a high potential for abuse which may lead to severe psychological or physical dependence.
Examples of Schedule II narcotics include: hydromorphone (Dilaudid®), methadone (Dolophine®), meperidine (Demerol®), oxycodone (OxyContin®, Percocet®), and fentanyl (Sublimaze®, Duragesic®). Other Schedule II narcotics include: morphine, opium, codeine, and hydrocodone.
Examples of Schedule IIN stimulants include: amphetamine (Dexedrine®, Adderall®), methamphetamine (Desoxyn®), and methylphenidate (Ritalin®).
Other Schedule II substances include: amobarbital, glutethimide, and pentobarbital.
Schedule III/IIIN Controlled Substances (3/3N)
Substances in this schedule have a potential for abuse less than substances in Schedules I or II and abuse may lead to moderate or low physical dependence or high psychological dependence.
Examples of Schedule III narcotics include: products containing not more than 90 milligrams of codeine per dosage unit (Tylenol with Codeine®), and buprenorphine (Suboxone®).

Examples of Schedule IIIN non-narcotics include: benzphetamine (Didrex®), phendimetrazine, ketamine, and anabolic steroids such as Depo®-Testosterone.
Schedule IV Controlled Substances
Substances in this schedule have a low potential for abuse relative to substances in Schedule III.
Examples of Schedule IV substances include: alprazolam (Xanax®), carisoprodol (Soma®), clonazepam (Klonopin®), clorazepate (Tranxene®), diazepam (Valium®), lorazepam (Ativan®), midazolam (Versed®), temazepam (Restoril®), and triazolam (Halcion®).

Schedule V Controlled Substances
Substances in this schedule have a low potential for abuse relative to substances listed in Schedule IV and consist primarily of preparations containing limited quantities of certain narcotics.
Examples of Schedule V substances include: cough preparations containing not more than 200 milligrams of codeine per 100 milliliters or per 100 grams (Robitussin AC®, Phenergan with Codeine®), and ezogabine.
Schedule 1 drugs are considered highly addictive, dangerous, and have “no medical value.” You will notice that cannabis is still listed in this category, in 2018. Who are the fellow “schedule 1” neighbors of cannabis, according to the DEA? That’s right. Ecstasy. Methamphetamine. Peyote. Do you believe that cannabis belongs here?

The problem with classifying cannabis as a schedule 1 drug is that it severely limits the ability of scientists, physicians, and researchers to study it. We need solid scientific data to prove whether the anecdotal tales of pain relief, spasticity reduction, alleviation of anxiety and nausea, and other apparent benefits can be proven. This is all the medical community really wants, to be able to finally prove what we all know to be true already: That cannabis is indeed a powerful, extremely safe medication for a variety of ailments.
Let’s look at the statistics. How many people to do you think die of overdoses caused by opiates in the US? The opiate medications are the standard prescription pain relief drugs such as Norco, Vicodin, and Codeine. They are highly addictive, dangerous drugs that are responsible for numerous deaths every year.
According to the CDC, “analysis shows that 38,329 people died from a drug overdose in the United States in 2010, up from 37,004 deaths in 2009. This continues the steady rise in overdose deaths seen over the past 11 years, starting with 16,849 deaths in 1999. Overdose deaths involving opioid analgesics have shown a similar increase. Starting with 4,030 deaths in 1999, the number of deaths increased to 15,597 in 2009 and 16,651 in 2010.
In 2010, nearly 60 percent of the drug overdose deaths (22,134) involved pharmaceutical drugs. Opioid analgesics, such as oxycodone, hydrocodone, and methadone, were involved in about 3 of every 4 pharmaceutical overdose deaths (16,651), confirming the predominant role opioid analgesics play in drug overdose deaths.” (CDC, 2015.)
What drug will not be found on a single overdose death from this same year? That’s correct. Cannabis.
Let’s look for a moment at deaths related to alcohol in the same year:
“Excessive alcohol use led to approximately 88,000 deaths and 2.5 million years of potential life lost (YPLL) each year in the United States from 2006 – 2010, shortening the lives of those who died by an average of 30 years.1,2 Further, excessive drinking was responsible for 1 in 10 deaths among working-age adults aged 20-64 years.” (CDC, 2015.)
Again, what drug was not responsible for a single death? Cannabis.
What does this have to do with MS?
Sativex is a currently approved drug in several countries, used for multiple sclerosis related spasticity and pain. It is a “high CBD” spray that is used sublingually, or under the tongue, several times daily. It has been shown to dramatically reduce pain and muscle spasm for MS patients around the world, but patients in the US have no access to it. Does this make any sense to you?
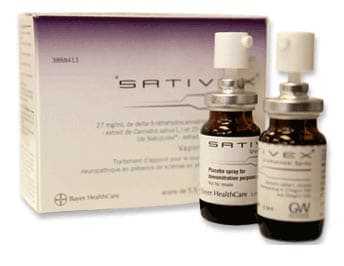
I’ve become a person who believes in the potential of medicinal cannabis to dramatically improve the lives of MS patients. I encourage everyone to write to local and national representatives, asking for the opportunity to try this medication that is so widely available in other countries, including Canada. It makes no sense to me that we are being prevented from trying these options, and instead handed opiates and benzodiazepines, which have proven their deadly risks.